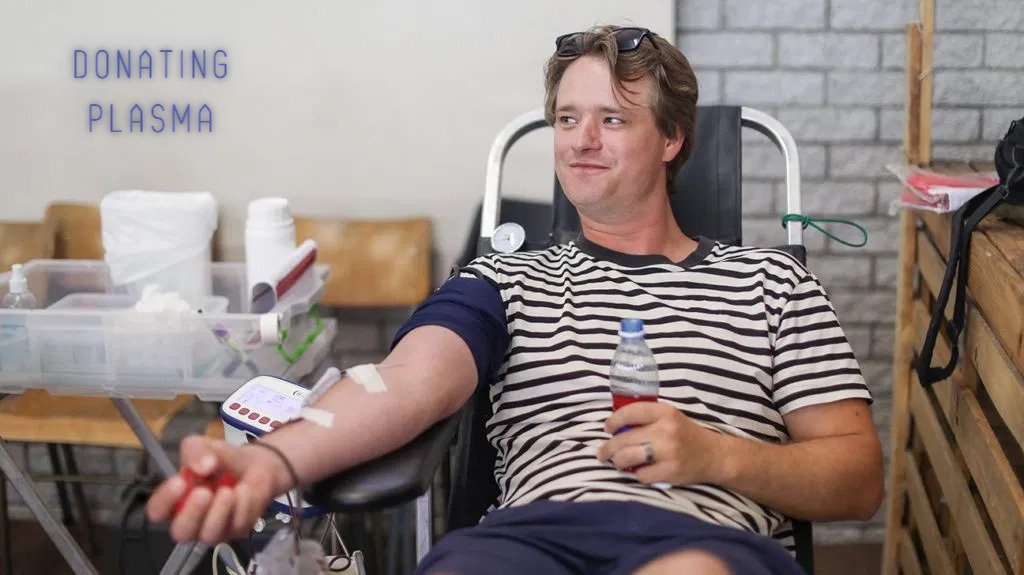
In order to donate plasma, you must be in good health. There are certain drugs and conditions that disqualify people from giving blood, however. Viral hepatitis A, B, or C is an example. Other medications that disqualify you are hemophilia and chronic bleeding disorders, including HIV. HIV-positive people cannot donate plasma. People with hepatitis C are also disqualified from donating blood.
Effient
If you’re considering donating plasma, be aware that certain drugs may affect your eligibility. These include insulin from cows, growth hormone from human pituitary glands, and experimental medicines. You may not be able to donate blood if you’re taking Hepatitis B immunoglobulin, Feldene, Plavic, or Ticlid. These rules are in place to protect both the donor and recipient.
Other medications may also disqualify you from donating blood. People taking certain prescription medications or OTC products, such as Aspirin, may be ineligible to donate platelets or whole blood. Anticoagulants are drugs used to prevent blood clots and strokes. These drugs may interfere with the ability of the blood to clot, which can cause excessive bleeding and bruising.
The statistical probability of complete loss of consciousness is 0.1%. One case occurs every 1,000 donors. More than half of those who experience this type of reaction reported mild symptoms, such as pallor, sweating, or a cold feeling. Only four people experienced severe reactions, including vomiting, loss of consciousness, or convulsive syncope. If you’re not certain about your ability to donate blood, you can find out more about it by visiting a local donation center.
Another reason why Effient and other similar medicines disqualify you from donating plasma is that they cause abnormal blood clotting. People taking these medications need to wait seven days before donating blood. This means that they cannot donate platelets for at least a month after the last dose of their medications. But the longer the waiting period is, the better. The medication also prevents your body from producing blood, which helps save lives.
The coronavirus is still affecting blood drives across the country. While it’s not deadly, the virus can be transmitted during blood transfusions. That’s why it’s important to make sure your blood donation is safe before donating. A person who has HIV should contact their local health department for advice. It’s important to have a clean blood check before you donate plasma.
Brilinta
The benefits of plasma donation and Brilinta therapy are many. This medicine attaches to platelets, which are responsible for clotting and preventing serious cardiovascular events. It works by preventing platelets from adhering together and activating. This drug is a good choice for people with platelet deficiency because it decreases the risk of clotting, which can lead to heart attack or stroke.
Among the side effects of Brilinta is a cough. Your doctor can help you decide whether this is a normal side effect or if you should seek medical attention immediately. If you cough blood or phlegm that looks like coffee grounds, call your doctor. If you develop a cough due to Brilinta, you should discuss other medications with your doctor. Your doctor can also give you other blood thinners or other blood-thinning medications that can reduce your risk of clots or heart attacks.
Some of the side effects of Brilinta include shortness of breath, nausea, and fatigue. Some people may even experience fainting or heart problems. If you are pregnant or lactating, you should talk with your doctor before taking Brilinta. Breastfeeding is not recommended while you are receiving Brilinta treatment, and it may pass into the breastmilk of a nursing mother. Although animal studies don’t always predict human results, you should consider the risks and benefits of this therapy before making a decision.
Patients on blood thinners like Brilinta and Effient must wait at least 7 days after their last dose before donating blood. If you’re on these blood-thinning medicines, you should not donate platelets or plasma. These drugs may cause abnormal blood clotting. For example, Brilinta may decrease your risk of a heart attack by 17 percent. Aspirin is a medication used to treat deep venous thrombosis and atrial fibrillation.
Although diarrhea was not a common side effect of Brilinta during the clinical trials, it is an uncomfortable side effect that can make you feel dizzy or faint. Diarrhea may also be a sign of other health problems. Consult a doctor immediately if you experience diarrhea, and call 911 if you experience bloody diarrhea or stools. You should also consult your doctor if you have bleeding in your stool or other symptoms of a heart attack.
Teriflunomide
Teriflunomide inhibits the proliferation of T cells, and it is a common anti-cancer drug. The drug causes cell cycle arrest in the early S phase without inducing apoptosis. It also inhibits the de novo synthesis of pyrimidines and DHODH, two enzymes that help the immune system fight infections. It is important to note that this drug should not be administered to people with HIV, because it is associated with increased risk of infection and malignancy.
However, pregnant women should avoid AUBAGIO, as it can cause teratogenic or embryofetal harm in a pregnant woman. Although it was not proven to cause any fetal harm in animal studies, teriflunomide exposures in plasma were significantly lower than the amount found in human plasma. Pregnant women should discontinue AUBAGIO and consult a medical professional if they develop any of these signs. Women should also avoid accelerated elimination of teriflunomide while receiving AEP.
Researchers have used a rat model to study the interactions between teriflunomide and the disease-modifying effects of other drugs. The animal model mimics the pathophysiology of RRMS and limiting unwanted immune responses is vital. By inhibiting DHODH, teriflunomide reduces the proliferation of B and T cells, which are critical cellular mediators of the disease. Teriflunomide also inhibits dihydro-orotate dehydrogenase (DODDH). The inhibition of this enzyme decreases the number of activated lymphocytes.
In mice, teriflunomide treatment delayed the production of antiviral antibodies, and was associated with a transient increase in viral load in the central nervous system. It was associated with a comparable risk of infections and malignancies. While teriflunomide inhibits immune activation, it does not disrupt pathogenic or protective immune processes. However, a potential connection between the drug and plasma donation should be investigated further.
Although the study was small, the results suggest that teriflunomide reduces the risk of sustained accumulation of disability for patients with relapsing-remitting MS. It also reduced the use of corticosteroids. It reduced the relapse rate to a minimum of 22.3%. In fact, the drug is often prescribed to people with relapsing-remitting MS.
COVID-19 vaccine
A recent vaccine for the COVID-19 virus has resulted in an unexpected problem for plasma donors. The vaccine completely removes the antibodies to the COVID virus, making you ineligible for plasma donation. However, there is hope for patients undergoing COVID-19 treatment. Donating plasma to help these patients is an important part of surviving the virus, but it is also a risk.
Although the CDC and FDA issued guidance on the use of donated plasma, the World Health Organization has prohibited this practice. The Red Cross, meanwhile, still asks donors to donate blood and plasma. The organization says it acknowledges the new FDA guidance and will continue to accept people who have had the vaccine, as long as the patient has recovered from the virus. Red Cross officials said they will continue to evaluate the timeline and feasibility of this change, in the face of changing hospital needs.
In addition to the risk of COVID-19, there are other factors that may prevent you from donating plasma. If you have a history of the disease, or you are unsure of whether you are vaccinated, don’t donate plasma until your doctor has cleared you to donate. This vaccine can cause serious side effects, including kidney failure, liver disease, and cancer. Donating plasma is a noble and life-saving act.
After receiving the COVID-19 vaccine, you may still be eligible to donate blood. As long as you are symptom-free and otherwise healthy, you can still donate blood after receiving the COVID-19 vaccine. However, you should remember that the vaccine does not lower the level of antibodies that protect you against the COVID-19 virus. In addition to this, you should contact your doctor or Red Cross if you have any questions.
People with active tuberculosis, HIV/AIDS, and live shingles virus must wait for 21 days after receiving the live shingles vaccine. Other vaccines have a deferral period of four weeks or more. The American Red Cross recommends that a person who has received a live shingles vaccine defer donating blood for four weeks. In addition to these precautions, COVID-19 vaccination may cause an unnaturally long deferral period from blood donation.